CLIENTS
MEDC. PARTNERS’ CLIENT PORTOFOLIO
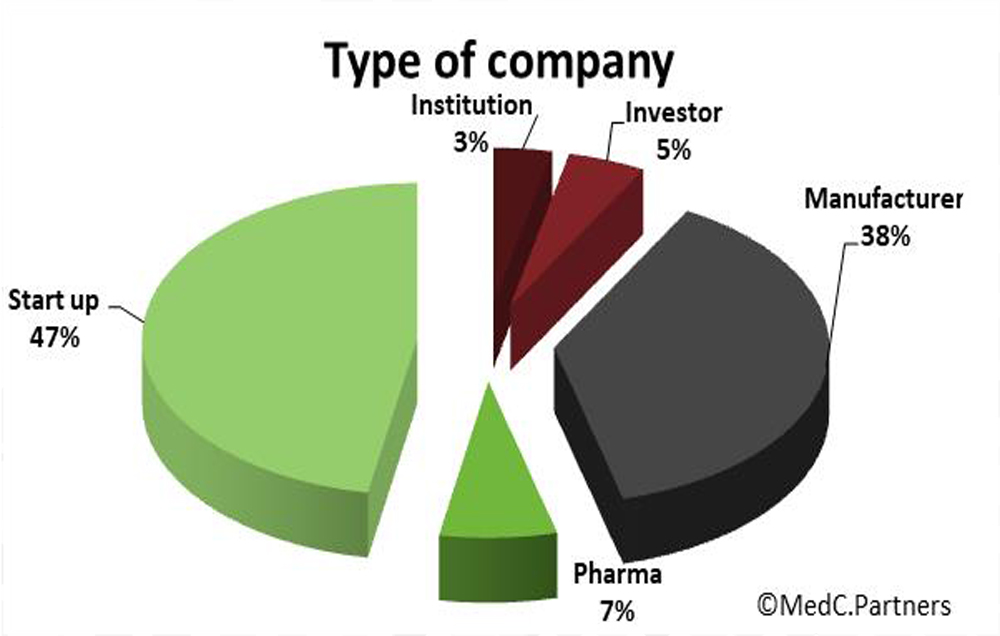
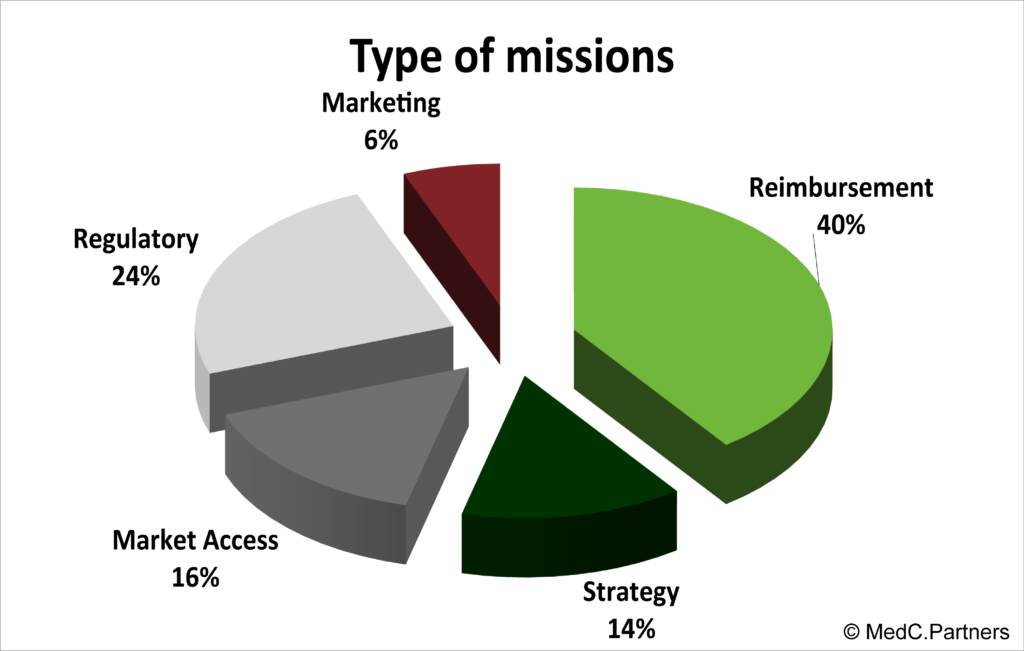
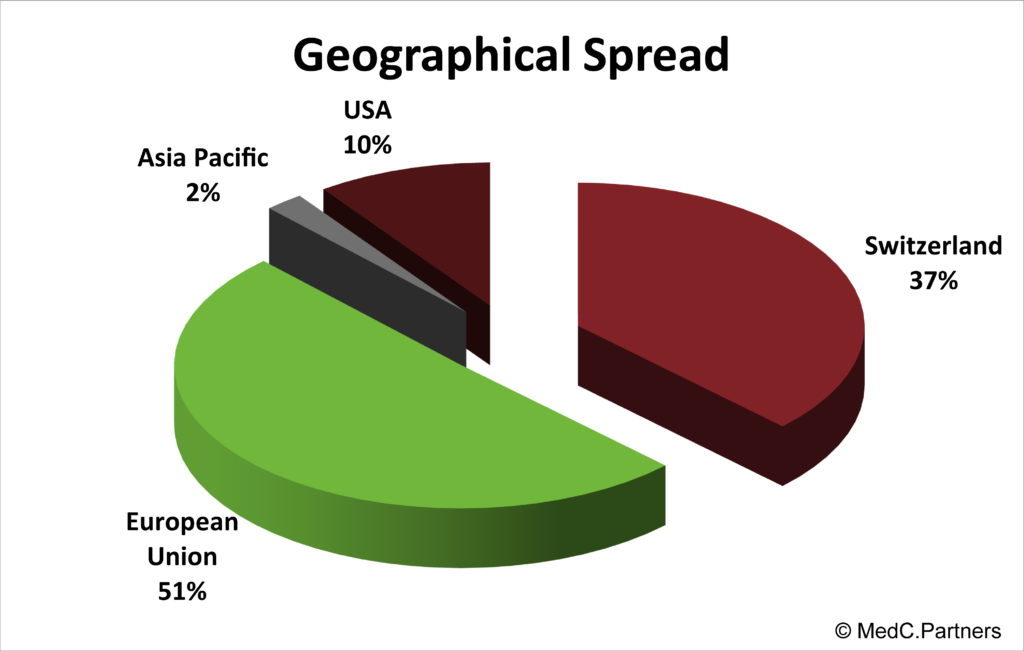
MedC. Partners thank their clients for their confidence and their long lasting relationship. They are located in Europe and North America and have multiple profiles.
MISSIONS
MedC.Partners cover a large spectrum of specialties and in particular: conventional and laparoscopic general surgery, cardiac surgery and MICS, interventional cardiology and neurology, urology and gynecology, diagnostic, sport and aesthetic medicine with a specific experience in single use, implantable and energy-based devices.
REIMBURSEMENT STRATEGY
Reimbursement
- Elaboration of an optimized reimbursement strategy for medical devices in Europe
- Reimbursement landscape based on MedMAP™, a Market Access programme developed by MedC Partners
- Reimbursement: Strategy, filing, submission, negotiations
- Health economics: economic story preparation (value for health), modelling, cost- effectiveness study
- Value pricing: Optimization of price within the healthcare environment
- Clinical: Clinical assessment for coherence with reimbursement strategy
- Face-to-face meetings with experts: authorities, payers
- Interviews with healthcare stakeholders: patients, doctors, payers
MARKETING STRATEGY
Business and strategic evaluation
- Evaluation of new projects in the medical business and extrapolation of the current tendencies in future market opportunities
- Analyzing of new business opportunities and current work on establishing a spin off company based on new indications of the core technology
- • Preparation of enhancement a Business Plan in order to submit it to a Venture contest
- Business opportunity analysis for a urinary bulking agent
- Development of a pre-market analysis for the European breast cancer detection market including the regulatory and reimbursement approach
- Introduction to investors
Operational marketing and distribution
- Marketing: Marketing plan, market assessment, positioning, customer profiling
- Business: Business plan, project assessment, due diligence, market survey
- Optimization of medical device strategy for pharmaceutical industry
Strategic and marketing consulting
- Search and optimization of distribution in France
- Strategic coaching
- Elaboration of an optimized strategy to distribute a disposable medical device in Europe
- Marketing tools development, brochures, product manual, events management
- Development of marketing and communication strategy
REGULATORY STRATEGY
Regulatory Affairs
- Borderline drug/device status
- Combination products (device with ancillary substance)
- Technical and strategic consulting on process of sterilization process
- Technical and strategic consulting on implantable device development and analyzing of Europe/USA normative framework
- Operational consulting on quality assurance system for development, manufacturing and marketing of a implantable medical device
- Construction of CE marking dossiers
- Qualification (evaluation) of a subcontractor
- Contribution to the implementation of required actions for the elaboration of a system of quality assurance system and construction of a technical dossier
- Construction of a technical and marketing dossier for a medical device allowing the valorization on a medical device offer to pharmaceutical laboratory
- Modification of manufacturing conditions of a medical device. Regulatory impact
TESTIMONIALS
Edwards Lifescience chose MedC. Partners to build and submit a registration file for a new cardiovascular procedure in Switzerland. We are very satisfied with the collaboration with MedC. Partners. We were particularly impressed with MedC. Partners’ ability to synthesize sets of complex clinical and medico-economic data into a readable argumentation. We were also pleased that they succeeded in having key players in the field to co-sign the filing.“
Edwards LifescienceDirector Reimbursement Northern Europe
“I have worked with MedC. Partners when I needed expertise to submit the reimbursement file of endografts for the treatment of AAA in France. MedC. Partners were able to step in the project immediately, they managed to carry it out in a record time, submitted the file on time to get the approval without additional question from the authorities. I really appreciated MedC Partner’s pragmatic approach, their ability to hear my needs, the quality of the interaction, and the team work.”
LeMaître VascularCountry Director
“We used the MedMap process to find out about the emerging market of continuous glucose monitoring in Europe. Starting from structured market research, the method helped us tremendously to identify the markets of high interest and shape our marketing strategy. MedC. Partners initially conducted a topline study for 10 European countries, which was followed on by a more in-depth analysis on the 4 most favorable countries. It was especially useful to visualize a complex and challenging situation in order to find the best solution for our company.“
CEOSolianis Monitoring AG
“We asked MedC. Partners to perform a topline MedMAP™ study. The MedMAP™ data and analysis were critical to become acquainted with the different European markets. Thanks to MedMAP™, we started our selection of priority countries that we would later focus on.”
CEOStentys
“Eclosion is the lake Geneva region life Science incubator and seed fund. We receive many applications we carefully evaluate and are routinely asking for expertises. MedC. Partners reviewed at our request a development plan for a cardiovascular device and validated the proposed budget, timeline, development milestones, which proved to be a valuable input. We were very satisfied by the level of professionalism performed by MedC. Partners.”
Director EclosionLife Science incubator
“G-Therapeutics contracted MedC. Partners to landscape the market access and reimbursement environment in 4 European countries for our innovative technology treating spinal cord injuries. MedC. Partners was able to collect a lot of data in a short period of time, and thanks to their method, called MedMap, they analyzed a very complex ecosystem. They helped us prepare an initial market access strategy by identifying the countries most favorable to our technology.”
CEOG-Therapeutics
“We started working with MedC. Partners before our technology was CE marked. Neolys in-vitro test can be applied to many therapeutic fields. Prioritizing the indications to focus on the ones offering the best return on investment was mandatory. We used the MedMap methodology. Going through the MedMap process and analyzing the result was very helpful to develop and adapt our market access strategy. We also used MedMap to identify the most favorable markets in Europe. It’s worth noting that MedMap has been a valuable tool internally for strategic decision as well as a concise, clear support to share our vision with different stakeholders. Overall our collaboration with MedC. Partners has been productive and contributed to the advancement of Neolys Diagnostics”.
CEONeolys Diagnostics